Orbital Diagram Chemistry Explained
Molecular orbital diagram example Chemistry: molecular orbital theor Define an atomic orbital.
Localized Bonding and Hybrid Atomic Orbitals
Atom orbitals arranged Orbital diagrams Orbital diagram carbon diagrams molecular orbitals o2 theory electrons atomic nitrogen sp3 hybridization molecules pairs do chemistry unbonded mo bonding
Chemistry: molecular orbital theor
Orbital diagrams — overview & examplesOrbitals hybrid hybridization atomic bonding chemistry molecular theory sp localized bond geometry orbital atom example using section structure involving central Orbital diagrams orbitals electrons monahan carolineOrbital diagrams — overview & examples.
Inorganic chemistryMolecular orbitals atomic orbital molecules socratic mo laid Electronic structure orbital diagrams chemistry pairing diagram spin box orbitals electrons energy boxes level spins represented atomic show ca subdivisionOrbitals hybridization hybrid chemistry orbital sp3 atomic hybridized three atoms sp each bond four valence equivalent blue red produces bonding.

1. electron configuration
Orbitals atomic chem chemistry configuration energy electronic electron shells electrons atom many first level levels capacity atoms four spin structureOrbitals chemistry electron atoms subshell order atomic configurations table number quantum periodic structure subshells electronic electrons energies which configuration energy Orbital atomic orbitals shapes given defineOrbital diagrams — overview & examples.
Electron configuration orbitals electrons orbit notation space pairsHow’re the orbitals in an atom arranged? Orbital molecularElectron configurations orbitals sublevel electrons each has line orbital sublevels levels hold box within.

Molecular orbital calculate atomic orbitals
Orbital diagram example 2s 2p 1sElectron configuration orbital chart diagram sublevel atom circle each wikimedia commons cc Atomic orbital molecular orbital diagram electron configuration aufbauOrbital diagrams monahan.
Hybrid atomic orbitalsEnergy orbitals orbital electron configuration notation chemistry diagram level electrons variation noble gas class charge effective nuclear fig various two Localized bonding and hybrid atomic orbitalsDrawing atomic and molecular orbitals diagrams for molecules.

Orbital diagrams tutor electron configuration
Electron configuration chartOrbital molecular molecule atomic diatomic orbitals homonuclear linear combination favpng Orbital diagrams — overview & examples2.2: electron configurations.
Electron configurationsOrbital molecular diagram bond mo nh3 identify ethene orbitals theory construct energy water h2 order then bonding non molecule electron Molecular orbital diagram atomic orbital molecular orbital theoryElectron orbitals atomic shell electrons levels subshell elements based table definition periodic structures process within.

Orbital electron orbitals atoms chemistry dimensional depicted
Orbital molecular diagram ethyne theory mo energy carbon electron ch orbitals molecules pictorial pairs fluoride chemistry sp below please hybridizationOrbital orbitals atomic chemistry shapes energy probability tutorial Orbitals, the basics: atomic orbital tutorial — probability, shapesDraw the molecular orbital diagram for the formation of $ n_{2.
Aufbau electron orbital principle atomic arrangement orbitals molecular atoms diagrama quantum configurations ck electrons principio chem 2s 2p libretexts 3sMolecular orbital diagram examples Orbital molecularOrbital molecular theory do diagram energy atoms combined different two when higher tell which inorganic hcl closed ago years so.
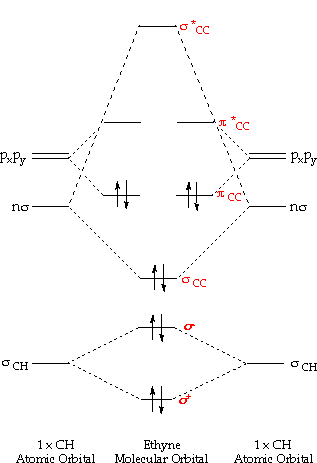
Chemistry: molecular orbital theor
Orbital orbitals definition atomic electron chemOrbital diagrams overview sulfur caroline monahan .
.


Electron Configurations | CK-12 Foundation
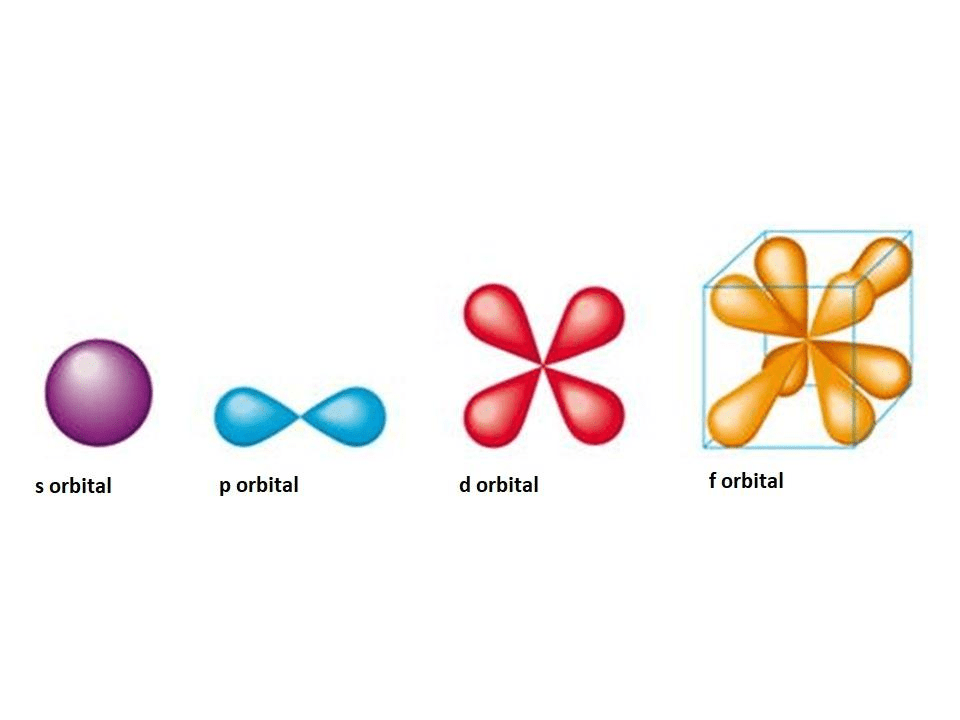
Define an atomic orbital.

Orbital Diagrams — Overview & Examples - Expii

Orbital - Definition, Diagram, Meaning - Study Chemistry

Localized Bonding and Hybrid Atomic Orbitals

Molecular Orbital Diagram Atomic Orbital Molecular Orbital Theory